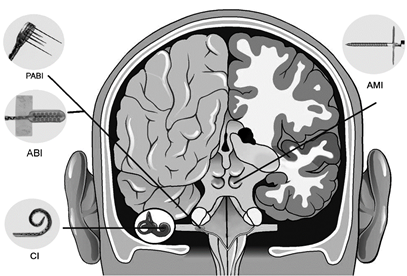
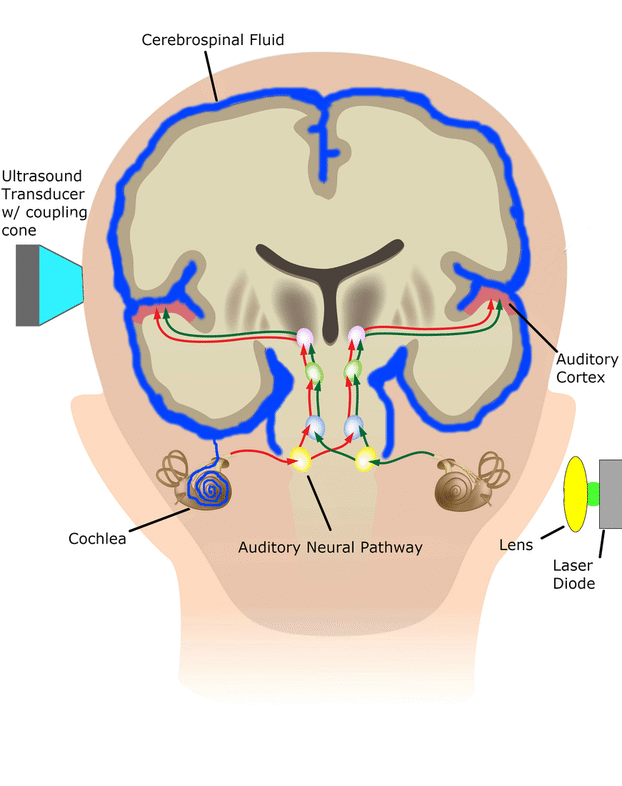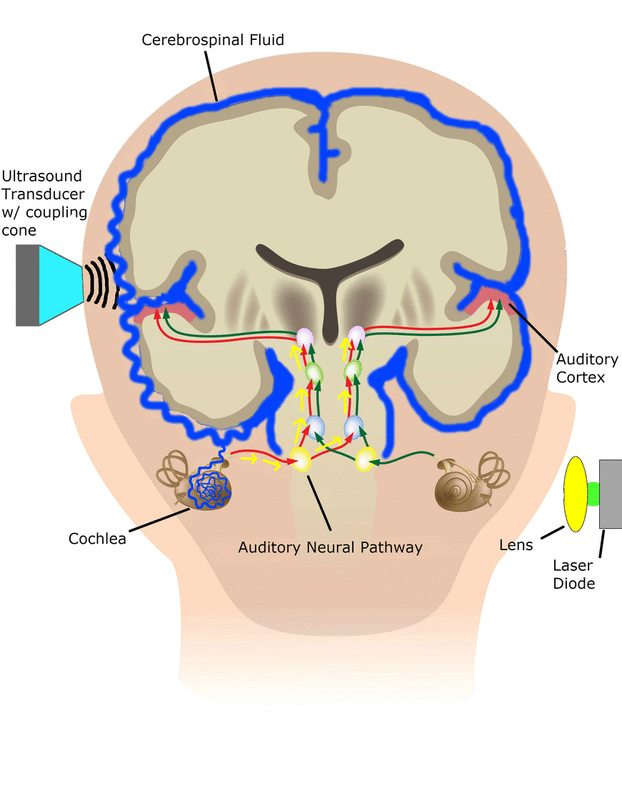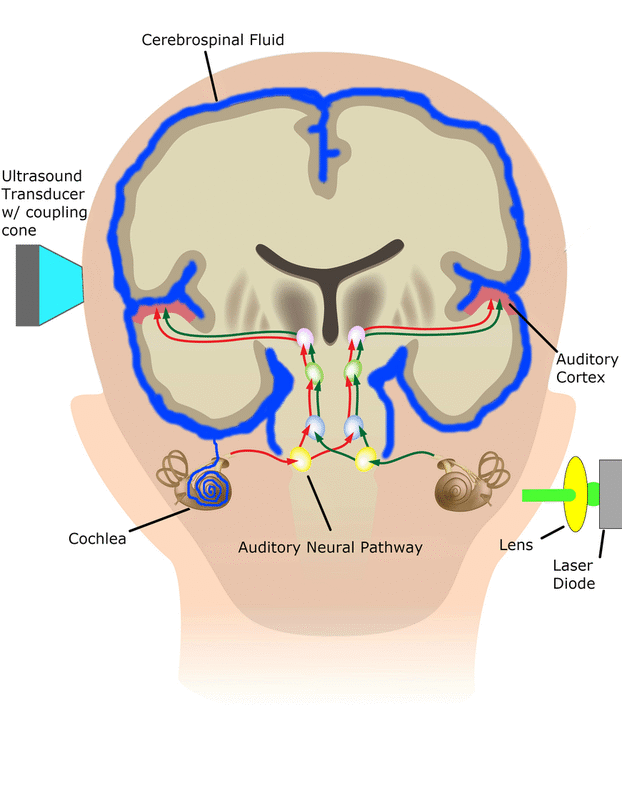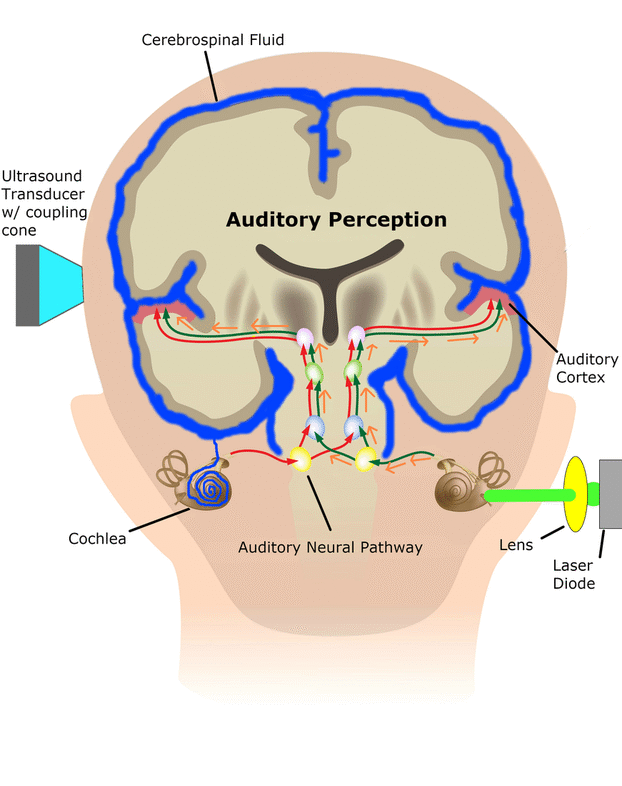
Central Auditory Prostheses
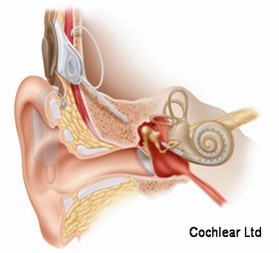
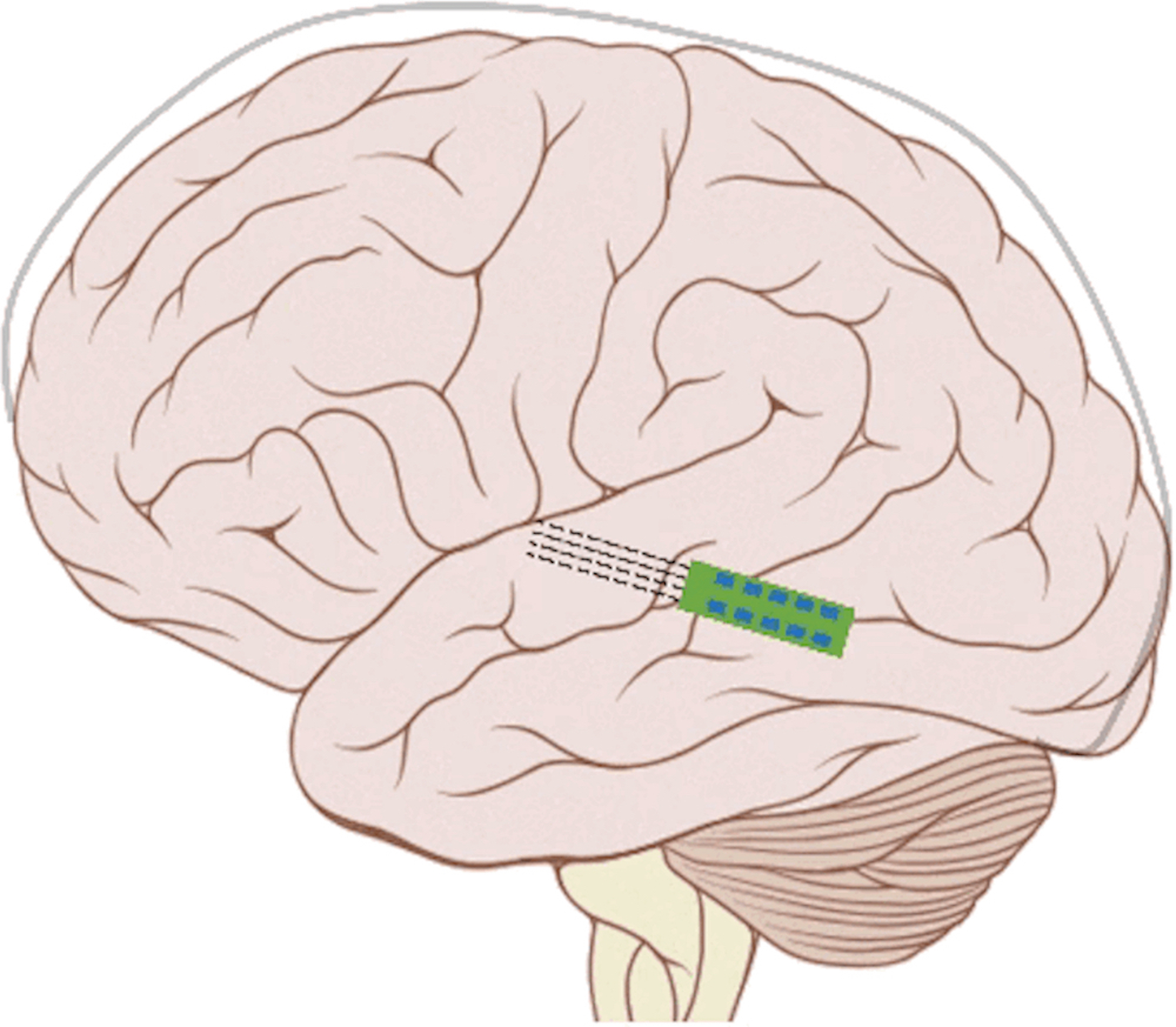
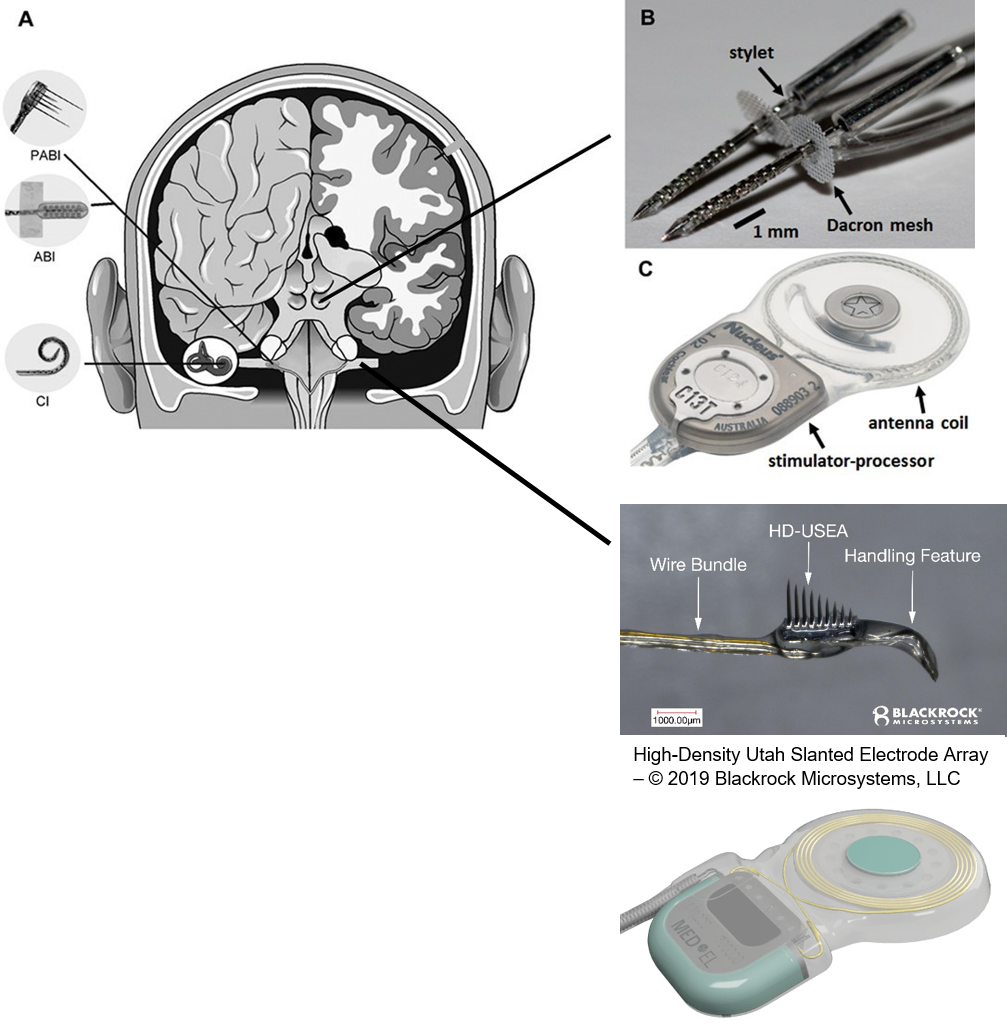
The cochlear implant is considered one of the most successful neural prostheses to date, which was made possible by visionaries who continued to develop the cochlear implant through multiple technological and clinical challenges. However, patients without a functional auditory nerve or implantable cochlea cannot benefit from a cochlear implant. Together with academic, clinical and industry partners (listed below), we have been developing and testing a new type of central auditory prosthesis for this group of patients that is known as the auditory midbrain implant (AMI) and is designed for electrical stimulation within the inferior colliculus. Although the auditory brainstem implant (ABI) is available to this group of deaf patients, its performance has been generally much lower than the cochlear implant. Therefore, a better solution is needed for these patients.
Auditory Nerve Implant
As mentioned above, the cochlear implant is considered one of the most successful neural prostheses to date; however, performance levels are still far from natural hearing. With recent advances in neural technologies and neurotological surgical approaches, there are greater opportunities for pursuing an intracranial auditory prosthesis that targets the auditory nerve between the cochlea and the brainstem (auditory nerve implant, ANI) to potentially improve hearing performance over current hearing treatment options (i.e. CI, ABI, AMI). A $10M 5-year NIH BRAIN Initiative grant has been awarded across five institutions and two medical device companies (listed below) to develop and translate a new ANI through pre-clinical studies (Years 1-3), and then implant the ANI in up to 3 deaf patients through a pilot clinical trial (Years 4-5).
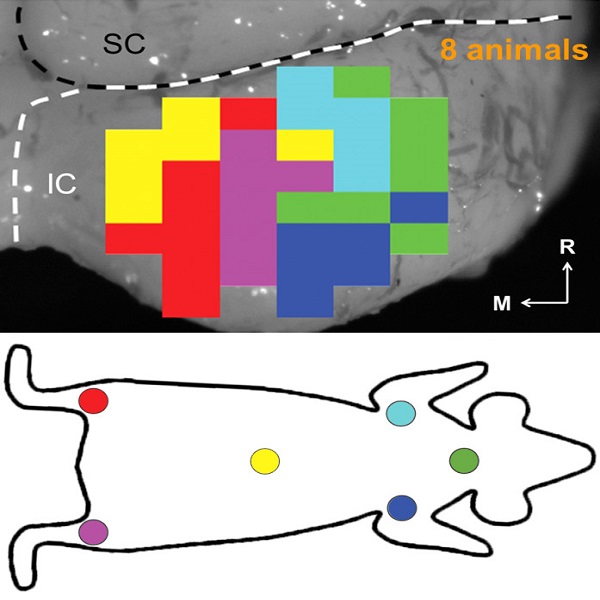
Our ANI research includes technology development and validation, behavioral/perceptual studies in animals and humans, and surgical/safety studies in animals and humans. The iterative design of the Utah electrode array for the ANI is being evaluated in human cadaver experiments, in which both retrosigmoid and translabyrinthine approaches are under consideration for implantation, to achieve proper cable anchoring and array insertion with a customized surgical inserter tool. The optimized array and cabling will interface with the MED- EL Synchrony implant. These components will be extensively tested for reliability, compatibility, and safety through benchtop testing and chronic animal experiments (in cats and non-human primates). Perceptual testing protocols and novel stimulation algorithms will be developed through simulation studies in normal hearing subjects and evaluated in CI subjects to compare the performance with that of ANI patients.
The overall goal of the ANI is to achieve more focused activation and a greater number of effective channels of information than the current standard of care, the CI. The major limitation of CI stimulation is the poor electrode-neural interface in which the electrode is placed in conductive fluid and separated via a bony wall from the auditory fibers causing current to shunt. The ANI will interface directly with the auditory fibers to overcome the poor electrode-neural interface that is seen with CI users and potentially provide better hearing outcomes including better perception of speech in noise and complex inputs (e.g. music appreciation). Initially, the ANI can be implanted in deaf patients who cannot be successfully implanted or sufficiently benefit from a CI due to anatomical distortions/obstruction of the cochlea or facial side effects. The success of this project will not only open new directions for auditory prosthetics, but also provided improved neural technologies for other clinical applications relevant to the BRAIN community.
Further details of the ANI research is provided by the NIH Reporter link below:
NIH Reporter - Link
Funding: National Institutes of Health BRAIN Initiative
Lead PI: Hubert Lim, PhD, University of Minnesota
Co-PIs: Thomas Lenarz, MD, PhD (Chair of Otolaryngology Department, Hannover Medical
School, Director of the German Hearing Center); Meredith Adams, MD (Associate Professor of
Otolaryngology, University of Minnesota); Andrew Oxenham, PhD (Professor of Psychology,
University of Minnesota); Loren Rieth, PhD (Faculty at Feinstein Institute for Medical Research);
Florian Solzbacher, PhD (Chair of Electrical & Computer Engineering, University of Utah); Rob
Franklin, PhD (Blackrock Microsystems).
Co-Is: Luke Johnson, PhD (Assistant Professor of Neurology, University of Minnesota); Geoff
Ghose, PhD (Associate Professor of Neuroscience, University of Minnesota); Sebahattin
Cureoglu, MD (Associate Professor of Otolaryngology, University of Minnesota); Amir Samii,
MD, PhD (Vice-President and Deputy Medical Director, International Neuroscience Institute,
Germany); Waldo Nogueira, PhD (Faculty at Hannover Medical School Hearing Center and
Hearing4All); Heiko von der Leyen, MD (Professor and Director; Hannover Clinical Trial Center); MED-EL
Auditory Midbrain Implant
Our AMI research has been performed over the past 18 years and has included physiology, behavioral/perceptual and safety studies in animals and humans as well as device and software development, allowing for translation of the AMI concept into human patients. The first AMI clinical study using a multi-site single-shank array was performed in 2006-2009. Although the single-shank AMI has achieved encouraging results in terms of safety and improvements in lip-reading capabilities and environmental awareness, it has not yet provided sufficient speech perception above what is possible with the current standard of care (i.e., ABI).
Since 2009, we performed further animal and human research, discovering that a two-shank AMI array can potentially improve hearing performance by targeting specific neurons of the inferior colliculus and minimizing unwanted suppressive effects induced by temporal stimulation patterns presented on individual electrodes. A new two-shank AMI device has been developed that is expected to improve hearing performance over the previous single-shank device and is currently being investigated in a clinical trial funded by the National Institutes of Health. The hope is that the two-shank AMI will serve as a better hearing alternative over the current ABI. We also envision that success with the AMI will open up new opportunities for central auditory prostheses to be more readily available to the general deaf patient population and provide a new generation of implantable devices that can achieve performance towards more natural hearing.
Further details of the AMI research and clinical trial are provided in these two review papers, NIH Reporter and clinicaltrials.gov listed below:
Lim HH, Shannon RV. "Two Laskers and Counting: Learning From the Past Enables Future Innovations With Central Neural Prostheses." Brain Stimulation, 8(3): 439-41, 2015. Invited Editorial Abstract
Lim HH, Lenarz T. "Auditory Midbrain Implant: Research and development towards a second clinical trial." Hearing Research 322: 212-223, 2015. Abstract
NIH Reporter - Link
Clinical Trial - Link
Funding: National Institutes of Health
Collaborators: Thomas Lenarz, MD, PhD (Chair of Otolaryngology Department, Hannover Medical School, Director of the German Hearing Center); Colette McKay, PhD (Professor, Bionics Institute, Australia); Amir Samii, MD (Vice-President and Deputy Medical Director, International Neuroscience Institute, Germany); Cochlear Limited (James Patrick, Chief Scientists - Emeritus); Hannover Clinical Trial Center (Heiko von der Leyen, Professor and Director)